Medical device development is rapidly moving toward smaller, smarter and more personalized solutions. To enable effective miniaturization and functional integration, whether for endoscopic tips, microfluidic implants, or ultra-thin dental restorations, manufacturers need production technologies that can reliably reproduce complex geometries at micron and sub-micron scales while meeting industrial tolerances and medical safety requirements.
At BMF we’ve focused on solving that exact problem.Our Micro 3D printing bridges the gap between lab prototypes and clinically viable products by delivering micron-level feature control for active components and tight-tolerance assemblies.Design freedom for internal channels, lattices, and multi-functional structures that are impossible or cost-prohibitive with conventional machining or molding.

Product highlight — microArch® S150: precision engineered for medical production
BMF’s latest industrial-grade system, the microArch® S150, is engineered to deliver stability, reproducibility, and user-focused safety features. Key advantages include:
- Sub-micron:25μm resolution for fine feature fabrication.
- Open Source Materials:Compatibility with high-performance resins and biocompatible materials.
- Automated industrial workflow:With no-leveling setup, intelligent interaction, and safe continuous operation.
- Healthcare-oriented environment controls:Including a built-in HEPA-13 filtration system and UV disinfection.
By combining precision and process reliability, the S150 shortens the transition from prototype validation to pilot-scale production, offering medical device companies an equipment platform designed for both innovation and scalability.

Materials innovation: expanding what’s manufacturable
We’ve developed material and process solutions that expand the palette of what can be produced via micro additive manufacturing.
BMF self-developed soluble sacrificial resins (SR) enables precision sacrificial tooling and mold workflows that make it possible to produce parts in PDMS, POM, PP, LCP and other polymer families via casting or injection over 3D-printed cores,opening routes to functional elastomers and engineering plastics that would otherwise be inaccessible by direct photopolymerization.

With superior biocompatibility, strength and wear resistance, zirconia has become a preferred material for dental and select high-value medical devices. BMF has achieved 80 μm ultra-thin zirconia veneers, significantly thinner than conventionally machined products. Such developments support minimally invasive dentistry while meeting global regulatory standards, including CFDA Class III certification and FDA approval.
These breakthroughs demonstrate that micro 3D printing ceramics are ready for clinical markets, offering batch-scale, high-performance components for regulated medical use.
From R&D to regulated manufacturing: partnerships and ecosystem focus
We believe the real value of micro 3D printing is realized when equipment, materials, process control and clinical partners work together. That’s why we’re building cross-sector collaborations—with universities, hospitals and industry partners—to solve scale-up, quality and regulatory challenges. Recent collaborations include deep work on multi-material and 4D printing technologies to explore next-generation implants and soft robotics, which could open new high-value medical applications.
BMF emphasizes that the full potential of micro 3D printing is realized when hardware, materials, and process control are aligned with clinical requirements. The company has expanded its R&D collaborations with universities, hospitals, and technology firms to tackle scaling, consistency, and regulatory challenges.
Recent initiatives include work with Shenzhen MultiMatter Science and Technology Co., Ltd. to explore multi-material and 4D printing applications, targeting advanced medical implants and soft robotics—fields with high potential for next-generation therapeutic solutions.
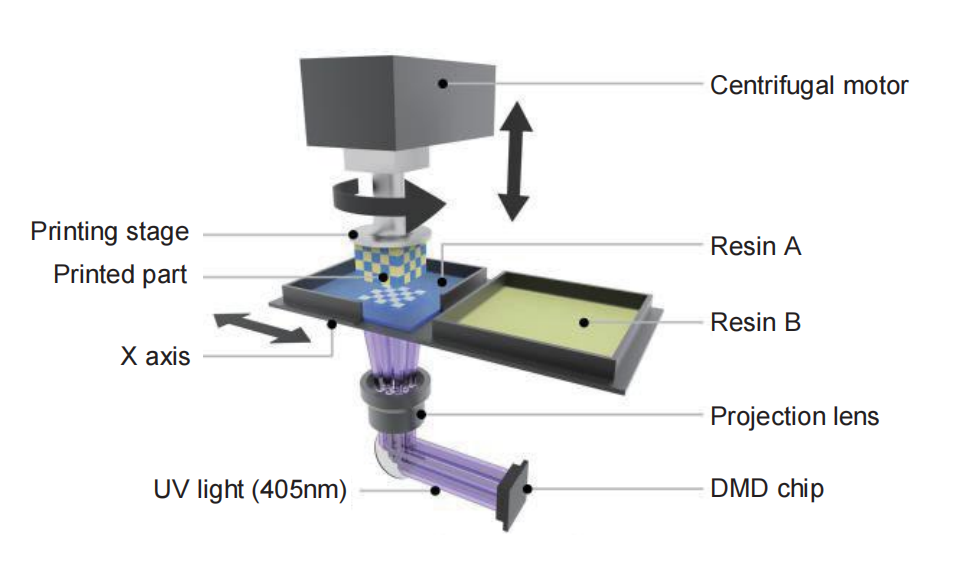
BMF continues to position micro 3D printing as a cornerstone for the future of high-end medical device manufacturing. Through continuous innovation in equipment, materials, and ecosystem collaboration, the company is committed to enabling safer, more precise, and more personalized medical technologies worldwide.
We look forward to partnering with you to turn ambitious device concepts into clinically meaningful, manufacturable products.

