A research team from the School of Pharmaceutical Sciences, Wuhan University has developed a pH-responsive bilayer microneedle patch that achieves ultrasound-activated antibacterial therapy and immune modulation, offering a powerful new non-antibiotic strategy for infected wound treatment. The study, titled “Three-in-one bilayer microneedle patch for ultrasound-activated antibacterial therapy and immune modulation in wound healing”, has been published in the Journal of Controlled Release.
The newly developed system, referred to as LFT/S-MN, integrates targeted bacterial capture, ultrasound-activated ROS generation, and acid-responsive anti-inflammatory drug release into a single microneedle platform. The patch features a bilayer structure, with a rapidly dissolvable base layer loaded with lactoferrin-based antibacterial nanoparticles, and a pH-responsive needle-tip layer encapsulating the anti-inflammatory agent spermidine (SPD).
When applied to infected tissue, the base layer quickly dissolves to release antibacterial nanoparticles that selectively bind to bacterial membranes. Under ultrasound stimulation, chemodynamic and sonodynamic effects trigger localized reactive oxygen species (ROS) generation, enabling rapid bacterial eradication. Meanwhile, the acidic wound microenvironment promotes sustained release of SPD, effectively regulating inflammation and supporting tissue regeneration.
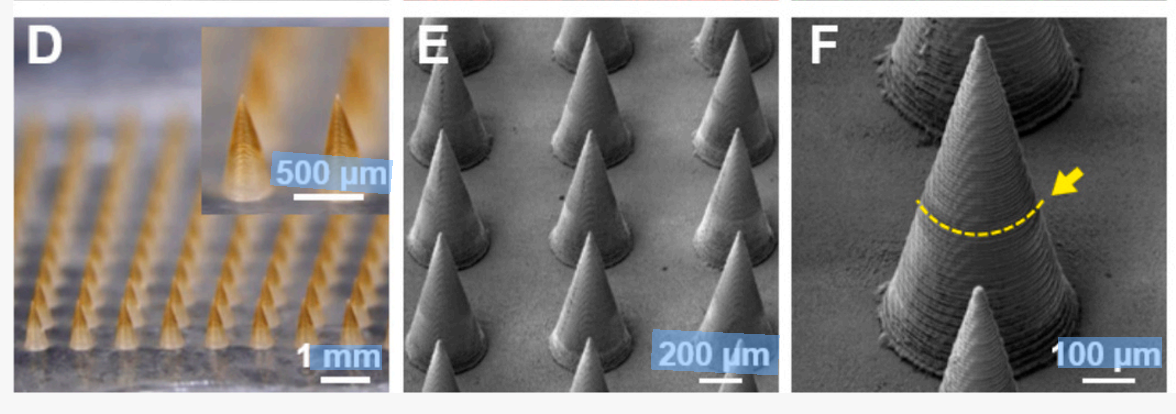
Crucially, the precise bilayer microneedle architecture was enabled by BMF’s projection micro stereolithography (PμSL) technology, using high-resolution printed molds to ensure excellent structural uniformity and mechanical strength. Each microneedle exhibits sufficient fracture force to reliably penetrate skin while maintaining controlled drug release behavior.
In both in vitro and in vivo assessments, the LFT/S-MN patch demonstrated over 99.9% antibacterial efficiency under ultrasound activation, along with significantly enhanced wound closure, collagen deposition, and angiogenesis in infected mouse wound models. No obvious systemic toxicity or body-weight abnormalities were observed during treatment.
This work highlights the transformative role of BMF’s micro 3D printing in advanced drug-delivery device manufacturing, and presents a promising new approach for non-antibiotic, precision-controlled antibacterial therapy and wound healing.
Explore the technical paper published in Journal of Controlled Release:
https://doi.org/10.1016/j.jconrel.2025.114375

